Project 1: Pre-Clinical Analysis of Inflammatory Bowel Disease
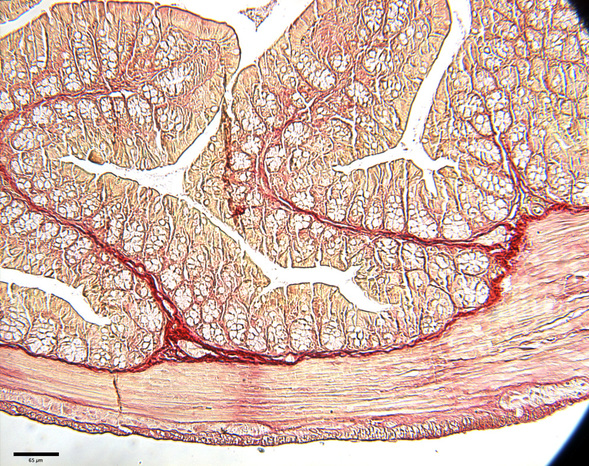
Inflammatory bowel disease (IBD) is a chronic intestinal disease characterized by inflammation of the gastrointestinal tract triggered most often by abnormal NFκB activation. The peak age of onset of IBD is between 15-30 years old and about 10% of cases are under 18 years old. Very early onset IBD represents children with a diagnosis < 6 years of age and has an incidence of of 4.37 in 100 000 and a prevalence of 14 in 100 000. Patients with long-lasting chronic IBD are at an increased risk for cancer - 5% increase per 10 years of chronic disease. IBD includes Crohn’s disease (CD) and ulcerative colitis (UC) and is highly prevalent in Canada. Current treatment is extensive and often requires lifelong immunotherapy. Currently, research suggests that IBD is caused by a combination of genetic predisposition, environmental influences, intestinal microbial disruptions, immunologic dysfunction and enhanced autophagic response. Over 160 susceptibility genes have been identified for IBD including a region on chromosome 3p21, the location of the Ras association domain family protein 1A, RASSF1A (or 1A). 1A is a tumor suppressor gene epigenetically silenced in a majority of human cancers (including inflammatory Hodgkin’s lymphoma and colorectal cancer [CRC]) resulting in its functional inactivation.
In addition, epigenetic silencing of 1A has also been detected in pre-condition diseases to cancer such as IBD and pancreatitis to suggest regulation of cellular homeostasis beyond a tumor suppressor gene. 1A has been demonstrated to associate with and promote TNF-R1-dependent cell death. We have evidence for 1A associations with Toll (TLR) and TLR molecular components (but not TNF-R1) that function to restrict NFkB activation. Thus, we would predict that the loss of 1A may result in abnormally high NFkB activity and increased inflammation. Using the dextran sodium sulphate (DSS) model to induce colonic irritation and damage and experimental colitis, DSS-treated Rassf1a knockout mice resulted in clinical symptoms of human colitis including increased intestinal permeability, enhanced cytokine/chemokine production, elevated NFkB activity, severe colonic epithelial cell injury and < 20% survival. In addition, DSS treatment of Rassf1a knockout mice was accompanied by increased DNA and oxidative damage, active autophagy and abnormal tyrosine (pY, not serine) phosphorylation of the Hippo transcription factor, YAP, and the obligate kinase of the nucleotide-binding oligomerization domain-containing protein2 (NOD2), receptor interacting protein kinase 2 (RIPK2, also known as RICK1 and RIP2 but herein referred to as RIPK2, unpublished observations). The NOD2/RIPK2 pathway has links to NFkB and the autophagic responses and thus are attractive targets to pursue for both acute and chronic inflammatory states that may lead to malignancy.
In addition, epigenetic silencing of 1A has also been detected in pre-condition diseases to cancer such as IBD and pancreatitis to suggest regulation of cellular homeostasis beyond a tumor suppressor gene. 1A has been demonstrated to associate with and promote TNF-R1-dependent cell death. We have evidence for 1A associations with Toll (TLR) and TLR molecular components (but not TNF-R1) that function to restrict NFkB activation. Thus, we would predict that the loss of 1A may result in abnormally high NFkB activity and increased inflammation. Using the dextran sodium sulphate (DSS) model to induce colonic irritation and damage and experimental colitis, DSS-treated Rassf1a knockout mice resulted in clinical symptoms of human colitis including increased intestinal permeability, enhanced cytokine/chemokine production, elevated NFkB activity, severe colonic epithelial cell injury and < 20% survival. In addition, DSS treatment of Rassf1a knockout mice was accompanied by increased DNA and oxidative damage, active autophagy and abnormal tyrosine (pY, not serine) phosphorylation of the Hippo transcription factor, YAP, and the obligate kinase of the nucleotide-binding oligomerization domain-containing protein2 (NOD2), receptor interacting protein kinase 2 (RIPK2, also known as RICK1 and RIP2 but herein referred to as RIPK2, unpublished observations). The NOD2/RIPK2 pathway has links to NFkB and the autophagic responses and thus are attractive targets to pursue for both acute and chronic inflammatory states that may lead to malignancy.
Project 2: Hodgkin’s
Lymphoma as a Model of Inflammation-driven Malignant Transformation
|
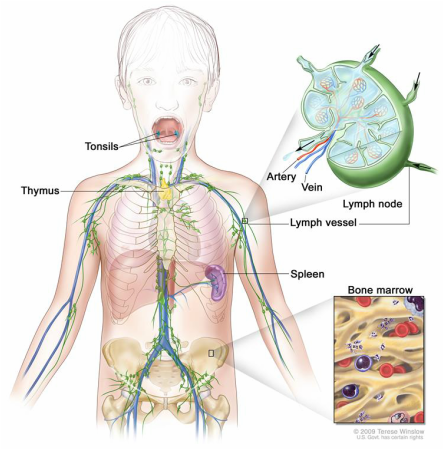
Lymphoma is a cancer of the lymphatic system whereby cells grow uncontrollably resulting in tumor formation. It can involve B or T lymphocytes and can begin in lymph nodes under the arms, in the groin, or in the abdomen or pelvis. Malignant lymphomas has increased dramatically with Hodgkin’s lymphoma (HL) as one of the most frequent lymphomas in the Western world, with an incidence of about 3 cases per 100,000 people per year. The childhood form of HL is more commonly diagnosed in the young adult (> 15 years of age) and older adults (>55 years of age). Furthermore, refractory HL patients or those who relapse several times have an extremely poor prognosis. HL is characterized by the presence of 1-5% Bcl-2 overexpressing neoplastic cells surrounded by >95% inflammatory cells as the major constituents of affected area. In this proposal, we will explore the use of specific modulators of inflammation and explore how they may reduce the inflammatory state and growth of HL cells. New therapeutic approaches are need to control the inflammation associated with HL.
|
Project 3: Immunomodulatory Control of Metastatic Breast Cancer
|
About 1/3 of all cancers arise from a state of chronic inflammation. Breast cancer has never be thought to be an “inflammatory cancer” but we have recent evidence to demonstrate that the triple negative breast cancer (TNBC) and inflammatory breast cancer (IBC, two highly metastatic forms of breast cancer) have significant up-regulation of cytokine/chemokine production and increased activity of mediators of inflammation. Since > 70% of breast cancer patients have epigenetic loss of 1A (a key negative modulator of inflammation and a tumor suppressor protein) and increased expression of RIPK2 (an important kinase in inflammation signaling), we have reason to believe that exploring ways to reduce the inflammation may be a new therapeutic angle for metastatic breast cancers. Our research interests will explore the characteristics of the inflammatory cells within metastatic breast cancer.
|
Contact us: [email protected]